COVID-19 vaccines are developed through a multi-step process that involves extensive research, testing, and regulatory approval. People all over the world have been eagerly waiting for a vaccine to be developed. But so far, there haven’t been any, none that are approved at least. However, plenty of drugs have been accepted by the WHO and the CDC to treat COVID positive patients. So, why hasn’t a successful vaccine been developed yet? How are vaccines developed and what makes vaccine development so complicated?
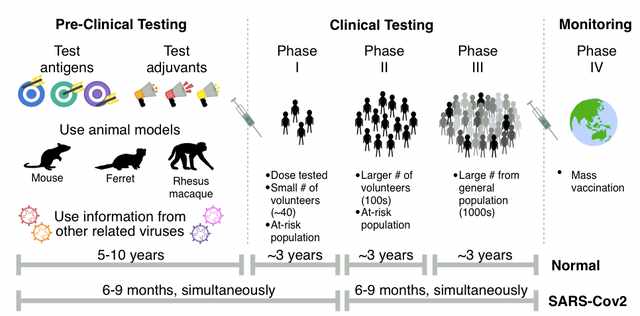
The stages of vaccine development are well defined. From the formulation of a potential vaccine to phased clinical trials, a vaccine has to go through multiple stages of development, checks, and downstream processing before they can be marketed or distributed for common use.
The first step of vaccine development happens in research labs or universities that are typically funded by either the government or some private pharmaceutical company. A potential vaccine is developed and tested on animals to measure their success(or failure). Then, cases of these controlled trials are published in peer-reviewed journals and conferences that are attended by physicians and scientists working in pharmaceutical companies who might approach the research labs to expand their research and focus on product development. However, most ideas never see fruition and fail in the next phase, i.e., clinical trials.
Nonetheless, a vaccine had to go through 3 rounds of clinical trials with positive outcomes in each. After the third phase of the clinical trials, a license is obtained and the vaccine is deemed successful. After obtaining the said license, the next step is the distribution of the vaccine. In the case of diseases that are non-emergent, distribution is generally not a concern. However, in the case of a raging global pandemic, distribution becomes complicated, mainly because of logistical, and ethical issues.
The misconception with vaccine development is that it is merely figuring out which vaccine is effective. In reality, that is only a small part of it(albeit a fundamental one). The WHO has hence set forth an estimated timespan of at least a year before a COVID-19 vaccine can be ready. Nonetheless, irrespective of when a vaccine is developed and is ready to be distributed, we must follow the old saying of “prevention is better than cure” diligently and scrupulously follow the guidelines laid down by both the WHO and the Ministry of Health and Family Welfare (MoFHW).